Time:2015-03-26
Mood disorders, such as major depressive disorders, are often accompanied by distributed system-level alterations in brain circuitry. There is increasing recognition that effective therapeutic interventions could reorganize structural and functional networks, resulting in correction of system-level abnormalities in the brain, eventually leading to complete rehabilitation. Converging evidence has demonstrated that a single subanesthetic dose of ketamine, a non-competitive N-methyl-D-aspartic acid (NMDA) receptor antagonist, produced a rapid-acting and long-lasting antidepressant response, attributable to changes in synaptic plasticity rather than simple antagonism of NMDA receptors. These features are extremely attractive for novel pharmacological strategies, and have inspired a flurry of preclinical and clinical studies on the neurobiological mechanisms of ketamine action. To date, however, the relevant brain circuits that underlie the long-term, efficacious action of ketamine remains unknown.
In a recent study, graduate student LV Qian and postdoc YANG Liqin under the supervision of Dr. WANG Zheng and Dr. HU Hailan, at the Institute of Neuroscience, Shanghai Institutes for Biological Sciences, CAS, conducted a randomized, placebo-controlled functional connectome study on anesthetized monkeys 18 hours after ketamine and saline administration. They applied graph theoretical analysis to quantitatively examine the overall topological organization of brain networks and used network-based statistics to characterize specific features within a network.
Ketamine intake induced persistent global reconfiguration of small-world properties, accompanied by large-scale downregulation of functional connectivity, most prominently in the orbital prefrontal cortex, subgenual and posterior cingulate cortices, and nucleus accumbens. Intriguingly, intrinsic connectivity within the medial prefrontal areas in the reward circuits were selectively downregulated. The global and regional changes in brain networks are precisely opposite to the maladaptive alterations observed in the depressed brain.
These findings demonstrated that local synaptic plasticity triggered by blockade of NMDA receptors was capable of translating into prolonged network reconfiguration in the distributed cortico-limbic-striatal circuit, providing mechanistic insight into developing specific loci or circuit-targeted, long-term therapeutics.
This work entitled “Large-scale persistent network reconfiguration induced by ketamine in anesthetized monkeys: relevance to mood disorders” was published online in Biological Psychiatry on February 28, 2015. This research was supported by the Chinese 973 Program (2011CBA00400), the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (XDB02030004), and the Outstanding Youth Grant of the National Natural Science Foundation of China (H.Hu).
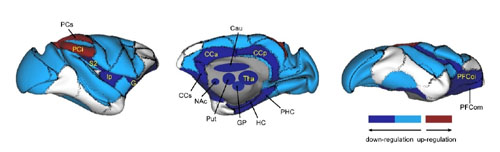
Figure. Overall illustration of affected macaque brain regions, shown by lateral (left), medial (middle) and ventral (right) views on the right hemisphere. The most prominent up- and down-regulated brain regions modulated by ketamine were labelled.
 附件下载:
附件下载: