Time:2018-09-18
The application of general anesthesia in clinical therapy has been an indispensable part of modern medicine for more than a century. Due to the complexity of the brain and the extensive actions of general anesthetic drugs, neural mechanisms underlying general anesthesia remain a mystery.
On September 18, an article entitled “The Locus Coeruleus Modulates Intravenous General Anesthesia of Zebrafish via a Cooperative Mechanism” was published in Cell Reports. This work was performed by Prof. YU Tian’s team at Zunyi Medical College and Dr. DU Jiulin’s lab at the Institute of Neuroscience, State Key Laboratory of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Science. By using larval zebrafish as a model, this study revealed that two commonly used intravenous anesthetic drugs, propofol and etomidate, suppress the excitability of locus coeruleus (LC) neurons via synergic mechanisms - inhibiting presynaptic excitatory inputs and inducing membrane hyperpolarization of these cells. This work indicates that the locus coeruleus - norepinephrine (LC-NE) system plays a modulatory role in both the induction of and emergence from intravenous general anesthesia.
The LC nucleus is a main site for synthesizing NE in the brain. Recent studies showed that activation of the LC-NE system in mice facilitated behavioral arousal from inhalational isoflurane-induced anesthesia. In contrast, inhibition of this system caused hypersensitive induction to and delayed the recovery from anesthesia induced by inhalational anesthetics. However, the role and synaptic mechanism about how intravenous anesthetics act on the LC-NE system are still rudimentary.
To tackle these questions, researchers first established an anesthesia zebrafish model via bath application of two intravenous anesthetic drugs, propofol and etomidate, and then identified the state of general anesthesia by examining animal’s spontaneous locomotion, and spinal nerve and brain electrical activities. Furthermore, local lesion of LC neurons via two-photon laser-based ablation or genetic depletion of NE synthesis accelerated the induction into and retarded the emergence from general anesthesia. Mechanistically, in vivo whole-cell recording revealed that both the anesthetics suppress LC neuron's activities through a cooperative mechanism - inhibiting presynaptic excitatory inputs and inducing GABAA receptor-mediated hyperpolarization of these neurons. These results indicate that the LC-NE system contributes to both the induction and emergence of intravenous general anesthesia via a cooperative mechanism, and highlight the application of the larval zebrafish model for studying neural mechanisms of general anesthesia.
This work was completed by DU Wenjie, with help from Drs LI Jia and ZHANG Baibing, under the supervision of Drs DU Jiulin, YU Tian and ZHANG Rongwei. This work was supported by Shanghai Science and Technology Committee (18JC1410100), Key Research Program of Frontier Sciences (QYZDY-SSW-SMC028) and Strategic Priority Research Program (XDBS01000000) of Chinese Academy of Science, and Natural Science Foundation of China (81571026).
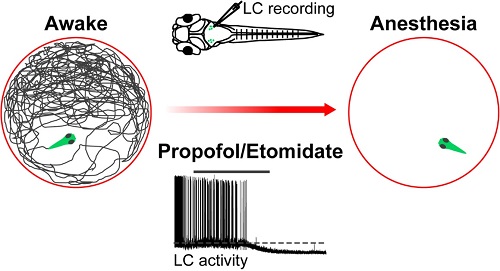
Figure legend: Propofol and Etomidate accelerate the induction into general anesthesia of zebrafish by suppress LC neuron's activities.
 附件下载:
附件下载: