Time:2019-02-28
On March 1st, a paper entitled “Cytosine base editor generates substantial off-target single nucleotide variants in mouse embryos” was published on Science, a breakthrough technique accomplished by the Institute of Neuroscience of Chinese Academy of Sciences (CAS), CAS-MPG Partner Institute for Computational Biology, Department of Genetics of Stanford University and Agricultural Genome Institute at Shenzhen. The researchers developed a method named “GOTI” (Genome-wide Off-target analysis by Two-cell embryo Injection) and applied to evaluate the genome-wide off-target effects induced by genome editing tools including CRISPR/Cas9 and base editors, and found that cytosine base editor induced substantial off-target single nucleotide variants (SNVs). Thus, GOTI significantly improved the sensitivity of off-target detection in the absence of prediction in advance and could also detect randomly generated off-target variants. Therefore, GOTI provides a new breathtaking method for the safety evaluation of genome editing tools, which could be taken as industry standard.
CRISPR/Cas9 is a new generation of gene editing tool that has been widely used. Since the appearance in 2012, it has aroused great attention for its efficiency and specificity. Scientists widely believe that clinical application of CRISPR/Cas9 and its derivatives will make great contribution to human health. However, the risk of off-target has been a serious concern since the advent of CRISPR/Cas9. The potential off-targets effects may cause many side effects including cancer if CRISPR/Cas9 and its derivatives are used for clinical application. Thus, a robust off-target detection method will be the key to the clinical application of CRISPR/Cas9 and its derivatives.
A variety of off-target detection schemes have been developed before. However, most of these methods relied on the prediction of off-target sites based on sequence similarity, or in vitro amplification which may introduce large amount of noise, making it difficult to separate off-target signals from background noise, especially single nucleotide variations. So whether CRISPR/Cas9 would induce off-target effects has been controversial. Therefore, a precise off-target detection method independent of computational prediction and with high signal-to-noise ratio is needed.
In order to achieve this goal, YANG Hui research team and collaborators established an off-target detection method named GOTI. When the mouse embryo developed into two-cell stage, a blastomere is edited and labeled with a red fluorescent protein (tdTomato) and the other kept unedited. The progeny cells of the edited and non-edited blastomeres were then sorted by FACS based on tdTomato expression in gene-edited cells at embryonic day14.5 (E14.5). Whole genome sequencing (WGS) was then performed on the tdTomato+ and tdTomato- cells respectively. SNVs and indels were called by three algorithms in the tdTomato+ sample, with the tdTomato- sample from the same embryo as the reference. This method avoids the noise problem caused by in vitro amplification. Moreover, since the experimental group and the control group are from the same embryo, the genetic background is completely identical, so the difference between the two cell populations can be considered to be caused by genome editing tools.
With the joint efforts of all members in YANG Hui Lab and the cooperators, the GOTI system was successfully established. By application of this system, the team members first tested the CRISPR/Cas9 system and found that the well-designed CRISPR/Cas9 did not have obvious off-target effects. This result ended the long-existed controversial of CRISPR/Cas9 off-target effects. The team also tested another CRISPR/Cas9-derived technology, BE3, which was reported to introduce point mutations, and no significant off-target problems have been found in previous studies. However, under the detection of GOTI, BE3 was found to generate substantial off-target SNVs, which were not predicted by traditional off-target prediction methods. They also found that some of the off-target sites appear on the oncogene and tumor suppressor genes, so the classic version of BE3 is of great concern for clinical application at present.
Based on the off-target detection by GOTI, the team found that some of the gene editing tools represented by BE3 have unpredictable off-target risks, allowing the world to re-examine the risks of these emerging technologies. More importantly, this work has established a genetic editing off-target detection method with higher precision, breadth and accuracy than before. GOTI can be applied to develop a new generation of genome editing tools with higher accuracy and safety and thus establish the new Industry Standard.
This work was accomplished by ZUO Erwei from the Institute of Neuroscience, CAS, SUN Yidi and WEI Wu from CAS-MPG Partner Institute for Computational Biology, and TANG Yuanlong from Agricultural Genome Institute at Shenzhen, under the direction of YANG Hui from Institute of Neuroscience, LI Yixue from CAS-MPG Partner Institute for Computational Biology and Lars M. Steinmetz from Department of Genetics of Stanford University and the European Molecular Biology Laboratory (EMBL). This work was also accompanied by other group members in the lab and the FACS , animal care, gene editing facility. This study was sponsored by R&D Program of China (2018YFC2000100 and 2017YFC1001302 to HY, 2017YFC0908405 to WW), CAS Strategic Priority Research Program (XDB32060000), National Natural Science Foundation of China (31871502, 31522037), Shanghai Municipal Science and Technology Major Project (2018SHZDZX05), Shanghai City Committee of science and technology project (18411953700, 18JC1410100) and NIH P01 Center grant (P01HG00020527 to L.M.S.).
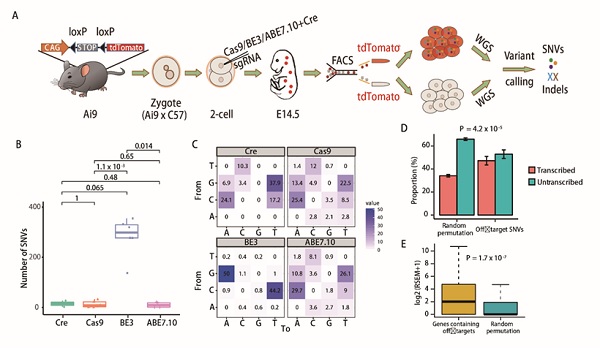
Figure 1. Schematic diagram of the “GOTI” technique and experimental results.(A) Experimental design. (B) Comparison of the total number of detected off-target SNVs. The number of SNVs for Cre-, Cas9-, BE3- and ABE7.10-treatedembryoswere14+/-12(SEM,n=2),12+/-4(SEM,n=11),283+/-32(SEM,n=6)and10 +/- 5 SNVs (SEM, n = 4), respectively. (C) Distribution of mutation types. The number in each cell indicates the proportion of a certain type of mutation among all mutations. (D) Off-target SNVs are enriched in the transcribed regions of the genome compared to random permutation. (E) Genes containing off-target SNVs were significantly higher expressed than random simulated genes in 4-cell embryos.
 附件下载:
附件下载: