Time:2023-04-10
A recent study published in Genome Biology used SLIM-seq technology to map the dynamics of RNA m6A modification during maternal-to-zygotic transition (MZT) in mice, and revealed the functions of m6A reading and writing on mouse preimplantation embryonic development through multi-omics analysis and CRISPR/Cas13d-mediated gene knockdown.
This research was conducted by LIU Zhen's research group and SUN Yidi's research group at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, in collaboration with Chen Li's research group at the Center for Single-Cell Omics, Shanghai Jiao Tong University School of Medicine.
All animal embryos pass through the MZT which is a fundamental and conserved process, during which the maternal environment of the oocyte transitions to the zygotic genome driven expression program. This process reprograms the terminally differentiated oocyte and sperm to a state of totipotency. MZT is initiated by maternal mRNAs and proteins that are present during the period of zygotic genome quiescence after fertilization. This is followed by a gradual switch to the zygotic genome activation (ZGA) and is accompanied by the clearance of maternal RNAs and proteins.
A key question for embryonic development is how the MZT is regulated. N6-methyladenosine (m6A) is the most prevalent internal modification present in the messenger RNA (mRNA) across higher eukaryotes. It affects the stability and translation of modified transcripts, providing a mechanism for coordinated regulation of groups of transcripts mediated by writer, eraser, and reader proteins during cell fate maintenance and transitions.
Using the SLIM-seq recently developed by Dr. ZHANG Haojian's group at Wuhan University (Yin et al., Cell Stem Cell, 2021), the researchers mapped RNA m6A dynamics during MZT in mice and found that m6A modifications can be maternally inherited or de novo gain during the ZGA. Analysis with translatome data published by Dr. ZHANG Yi's group at Harvard University (Zhang et al., Sci Adv) showed that a group of maternally inherited and persistent m6A+ transcripts were significantly enriched for translational signaling.
Further, using a recently developed ultrasensitive proteome technique (Gu et al., bioRxiv, 2023), the researchers found that these m6A+ transcripts showed elevated protein abundance, thus suggesting that m6A modifications on these transcripts could potentially regulate MZT by maintaining RNA stability.
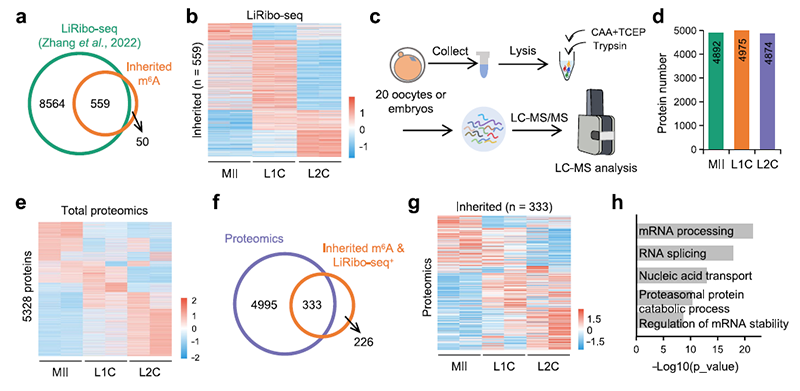
Figure 1. A group of maternally inherited and persistent m6A+ transcripts showed a significant translational signal and increased at protein level.
Furthermore, researchers screened for multiple m6A regulators in fertilized eggs of mouse using CRISPR/Cas13d technology, which has recently been shown to be used for embryonic gene silencing (Kushawah et al., Dev Cell, 2020), and found that knockdown of Ythdc1 and Ythdf2 significantly affected preimplantation embryonic development. Through transcriptome sequencing and RIP-qPCR experiments, the researchers confirmed that Ythdc1 can bind some maternal m6A+ transcripts and participate in their RNA stability regulation.
This study reveals the dynamics of m6A modification during mouse MZT, identifies a few of m6A regulators involved in preimplantation embryonic development, preliminarily elucidates the possible involvement of Ythdc1 in the stability regulation of maternally inherited m6A+ transcripts, and exemplifies the importance of multi-omics analysis in embryonic development investigations.
This work entitled "Reading and writing of mRNA m6A modification orchestrate maternal-to-zygotic transition in mice" was published online in Genome Biology on April 6, 2023.
Dr. ZHU Wencheng, Dr. DING Yufeng and graduate students MENG Juan and LIU Wenjun from the Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, and research assistant GU Lei from the Center for Single-Cell Omics, Shanghai Jiao Tong University School of Medicine are co-first authors of the paper. The research was supported by grants from the Ministry of Science and Technology, Chinese Academy of Sciences, Shanghai Municipality and China Postdoctoral Science Foundation.
Keywords: RNA m6A Dynamics; Maternal-To-Zygotic Transition
AUTHOR CONTACT:
LIU Zhen
Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, China.
E-mail: zliu2010@ion.ac.cn
 附件下载:
附件下载: