Time:2023-05-11
A new study published in Neuron revealed that cholecystokinin (CCK) neurons in the suprachiasmatic nucleus (SCN) play an important role in the regulation of the robustness of mammalian circadian clock. This study was performed by Dr. YAN Jun's lab at the Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences.
Circadian clock is an intrinsic time keeping mechanism evolved in animals to adapt to the 24-hour periodic environment on Earth. Suprachiasmatic Nucleus (SCN) consisting of roughly 20,000 cells has been known as the master circadian pacemaker to regulate a wide range of physiological processes and behaviors in mammals.
Environmental signals such as Light can enter SCN to entrain the circadian clock. On the other hand, circadian clock persists to function under different environments including seasons characterized by different photoperiods. The neural mechanisms underlying the plasticity and robustness of circadian clock are still unclear. Past studies of SCN have mainly focused on neuropeptide-releasing neurons including vasoactive intestinal polypeptide (VIP) and arginine vasopressin (AVP) neurons in the SCN.
Previously, Dr. YAN Jun's lab discovered a new neuronal subtype in the mouse SCN that highly expresses the cholecystokinin (CCK) through single-cell RNA sequencing and tissue-clearing assisted imaging (https://doi.org/10.1038/s41593-020-0586-x), but the precise function of SCN CCK neurons in the circadian rhythm was not known.
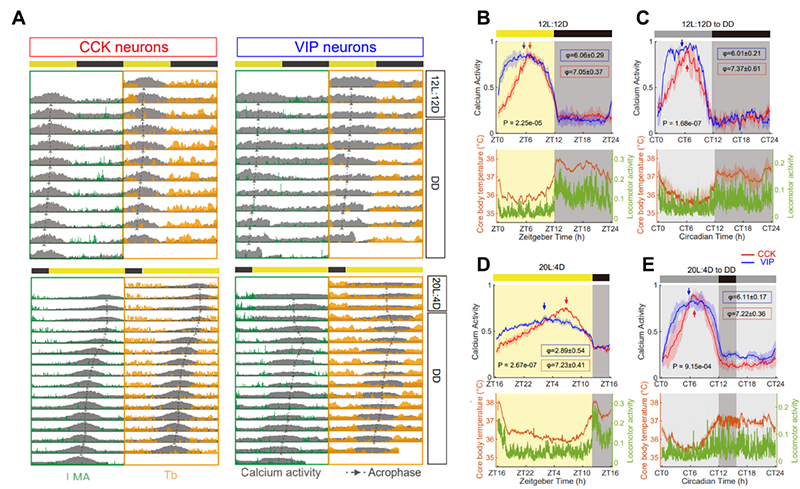
Figure 1. In vivo calcium activities of SCN CCK and VIP neurons under different photoperiods.
(A) Representative plots of in vivo GCaMP6s signals of SCN CCK and VIP neurons overlaid with locomotor activity and body temperature in the actogram under 12L:12D or 20L:4D. (B-E) The average circadian profiles of in vivo GCaMP6s signals in SCN CCK and VIP neurons under different photoperiods.
In order to investigate the circadian functions of SCN CCK neurons, the researchers monitored in vivo calcium activity of SCN CCK neurons and behavioral activities in free-moving mice (Figure 1). They found that the calcium activities of SCN CCK neurons peaked before the activity onset of mice under different photoperiods, and thus encoded the timing of the start of circadian behavioral activity. Under long-day photoperiod and constant light, the circadian rhythm of calcium activity of VIP neurons is significantly dampened while the calcium activity of CCK neurons remains oscillating. CCK neuron deficiency led to the shortening of free-running period, and the instability of circadian behaviors specifically under long-day photoperiod and constant light (Figure 2).
Furthermore, the researchers found that CCK neurons are not directly light sensitive, in stark contrast to VIP neurons that can be activated immediately upon light pulse. Optogenetic activation of CCK neurons can phase advance the circadian clock and counter the phase delaying effect of light, thereby stabilizing the circadian clock under long-day photoperiod. This suggests that CCK and VIP neurons have opposite functions in circadian clock. Combining single-molecule fluorescent in situ hybridization (smFISH) and optogenetic manipulation, the researchers found that there is a negative feedback interaction between VIP and CCK neurons in SCN, and the impact of VIP neurons on the SCN network was significantly weakened while that of CCK neurons was strengthened in long-day photoperiod, underlying the seasonal plasticity of SCN network.
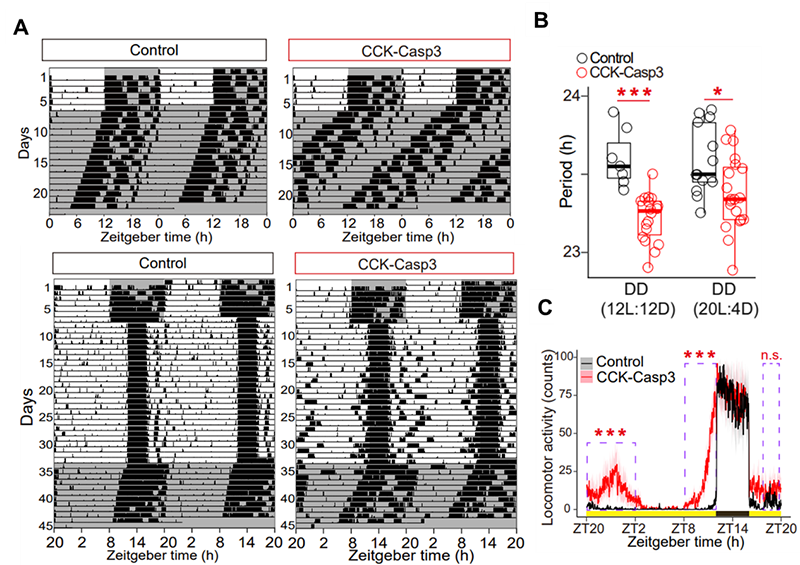
Figure 2. Circadian activity of CCK neuron deficient mice under different photoperiods.
(A) Representative double-plotted wheel running activity actograms of control and CCK-Casp3 mice under different photoperiods. (B) Free running periods were shortened in CCK-Casp3 mice. (C) Activity profiles of CCK-Casp3 mice showing advanced onset and delayed offset under 20L:4D.
Finally, the researchers found that the response of CCK neuron activity to the sudden change of light-dark cycle modeling the jet lag in mice was significantly slower than that of VIP neurons and the inhibition of CCK neurons can significantly shorten the time of recovery in jet lag (Figure 3). This result suggests that the slow shifting CCK neurons is the rate limiting factor of behavioral shift during the sudden changes of light-dark cycle.
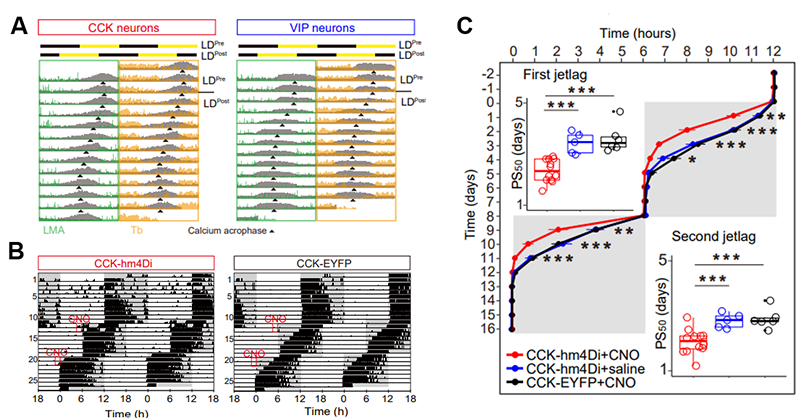
Figure 3. CCK neurons regulate the onset speed of 6-hour phase advance jet lag.
(A) Representative actograms showing the in vivo calcium signals (gray, double-plotted) of SCN CCK and VIP neurons. (B) Representative double-plotted actograms of wheel running activities of control and CCK-hm4D mice during 6-hour phase advance jet lag model. (C) Different rates of phase shifts of activity onset between control and CCK-hm4Di mice.
Built upon their previous work of single-cell reconstruction of mouse SCN, Yan’s Lab uncovered that the SCN neural network involving CCK neurons provides the neural basis for the robustness and plasticity of circadian clock in mammals (Figure 4). In the future, SCN CCK neurons may serve as a potential therapeutic target to treat the human health problems related to jet lag and shift work.
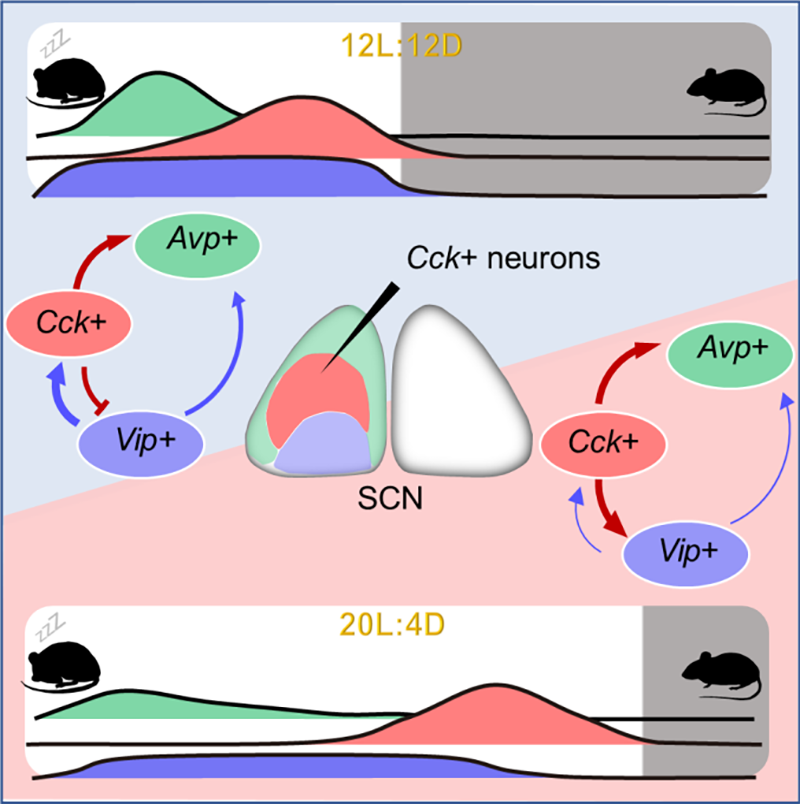
Figure 4. The neural networks of SCN Avp+, Cck+, and Vip+ neurons under different photoperiods (12L:12D vs. 20L:4D)
This work entitled "Cholecystokinin neurons in mouse suprachiasmatic nucleus regulate the robustness of circadian clock" was published online in Neuron on May 11, 2023.
This work was conducted by Dr. XIE Lucheng, Dr. XIONG Yangyang and Dr. MA Danyi, under the supervision of Dr. YAN Jun, with help from CHEN Jiu and SHI Kaiwen in Yan’s lab, and Dr. YANG Qiaoqiao in the department of neurosurgery in the Zhongshan Hospital of Fudan University. This work was supported by the funds from Ministry of Science and Technology, National Natural Science Foundation of China, Chinese Academy of Sciences and Shanghai Municipal Government.
Keywords: Neural Mechanism; Circadian Clock; Suprachiasmatic Nucleus; Jet lag; Shift work
AUTHOR CONTACT:
YAN Jun
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
E-mail: junyan@ion.ac.cn
 附件下载:
附件下载: