Time:2023-06-07
Recently, DU Jiulin’s research group at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, found the circadian regulation of synapse growth during neural development, taking the classic retinotectal synapse in zebrafish as an in vivo model implemented with the long-term time-lapse two-photon imaging. This study not only provides an important theoretical basis for the function of the circadian clock in regulating neural development, but also an important experimental basis for understanding the developmental rules of neuronal circuit formation.
The circadian rhythm is a timing phenomenon of organisms, usually affected by environmental cues such as light and temperature, and oscillating with about a 24-hour period. Under physiological conditions, the biological clock orchestrates the functions of neural, endocrine, cardiovascular, immune, metabolic as well as other biological systems. However, except for a few reports about its roles in regulating cell cycle in larval zebrafish, eclosion in Drosophila and kidney organogenesis in mammals, whether and how the biological clock regulates the early development of organisms are poorly understood.
To address this question, the researchers leveraged the zebrafish retinotectal synapse to study whether the biological clock regulates synaptogenesis, a fundamental developmental process for neural circuit formation. The zebrafish is an ideal model vertebrate for studying developmental processes in vivo, and its retinotectal system is a classic model for studying the neurite and synapse development. Researchers can use in vivo optical imaging technology to time-lapsely monitor the same object in the same living fish, thus making a detailed observation of developmental dynamics. In a previous study, the researchers created a transgenic zebrafish model specifically visualizing retinotectal synapses (Du et al., Sci Rep 2018). In this study, this model was applied to investigate the circadian rhythmicity of the synaptogenesis.
Researchers first entrained zebrafish larvae in normal light-dark conditions for four days after fertilization, and then performed the time-lapse imaging with a 6-hour interval for subsequent two days. Analysis showed that the rate of the increase in the number of synapses oscillated diurnally with faster increase during the day and slower increase at night. This oscillation persisted during free-running period in constant darkness or constant light, shifted its phase accordingly with reversed dark-light-cycle entrainment, while was disrupted in clocka-/- mutants, suggesting that this oscillation is regulated by the endogenous circadian clock system and thus is rhythmic. Furthermore, analysis of synapse growth dynamics obtained from the high temporal-resolution imaging, revealed that this rhythmicity arises primarily from the synapse formation rather than elimination. Then, the researchers found the disruption of the rhythmic synaptogenic rate by impairing the hypocretinergic (also known as orexin) system, suggesting the hypocretin signaling is required for this rhythmic development of synapses. Finally, disruption of this synaptogenic rhythm, by either the clock gene mutation or the hypocretin receptor mutation, resulted in the larger size and reduced complexity of the presynaptic retinal ganglion cells’ axon arbor, the decreased synapse number, and the decreased density and evenness of synapse distribution in axon arbor territories. In accordance with the enlarged axon arbor area, the visual receptive field of postsynaptic tectal neurons showed increase in the two mutants, indicating the visual acuity development is affected.
This study revealed the regulation of circadian clock on the early developmental process of organisms, expanding the functional spectrum of the circadian clock; on the other hand, it revealed a new regulatory mechanism underlying neural circuit development. The requirement of a sleep/wake regulator (hypocretin) in the maintenance of circadian synaptogensis rhythm provides insights into the interaction between the circadian clock and sleep-wake regulation, and into a new systematic clock output pathway.
This work entitled "Circadian regulation of developmental synaptogenesis via the hypocretinergic system" was published online in Nature Communications on June 2nd, 2023.
This study was mainly conducted by Dr. DU Xufei and Dr. LI Funing, under the supervision of Dr. DU Jiulin; Dr. DU Xufei is also a co-corresponding author; Dr. PENG Xiaolan made important contributions. Dr. YAN Jun from the Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, and Dr. WANG Han from the Soochow University are of great help. This work was supported by the Ministry of Science and Technology and the Chinese Academy of Sciences.
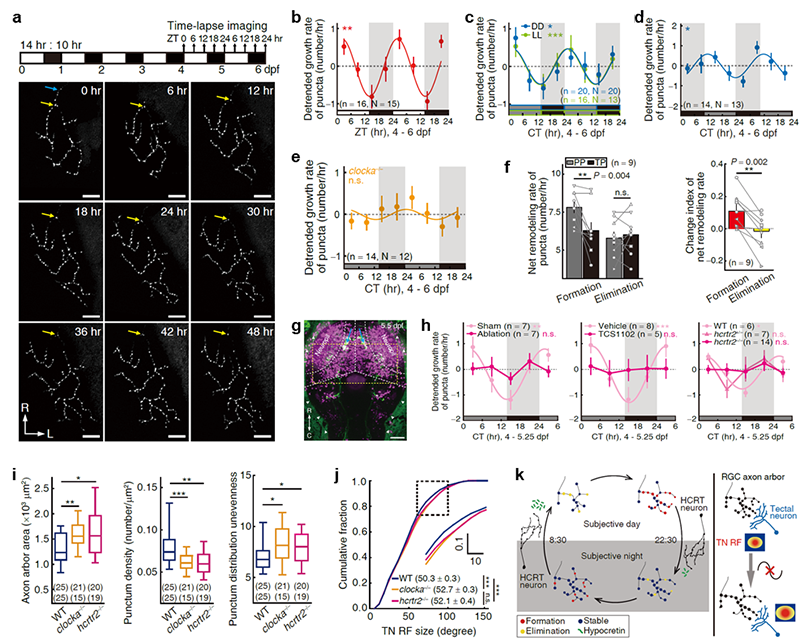
a Experimental design and a typical case of the time-lapse imaging of the presynaptic puncta of single retinal ganglion cell. b-d The growth rate of synaptic puncta under light-dark (b), constant dark or constant light (c), and reversed dark-light (d) conditions. e The diurnal oscillation is disrupted in clocka mutants. f Diurnal change of the synapse formation or elimination rate. g The expression pattern of zebrafish hypocretin neurons (green). h Disruption of the synaptogenic rate rhythm by impairing the visual receptive field of downstream visual tectal neuron hypocretinergic system. i,j Changes of the structure of presynaptic RGCs’ axon arbor and synapse arrangement (i), and of the visual receptive field of postsynaptic tectal neurons (j) in clocka mutants and hypocretin receptor (hcrtr2) mutants. k Working model.
https://www.nature.com/articles/s41467-023-38973-w
Contact:
DU Xufei
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
Keywords:
Synaptogenesis, Circadian rhythm,Hypocretin/Orexin, Zebrafish, Biological clock
 附件下载:
附件下载: