Time:2024-08-23
Recently, the research groups led by DU Xufei and Du Jiulin at the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology of the Chinese Academy of Sciences, developed an applicable and efficient circuit tracing tool for larval zebrafish. This study presents an important technological advancement of implementing recombinant rabies virus (RV) for trans-monosynaptic circuit tracing from single, genetically targeted neurons in larval zebrafish, providing a valuable tool for advancing neuroscience research with zebrafish as a model organism.
The larval zebrafish, with its tiny transparent brain, has been emerging as an ideal vertebrate model for systems neuroscience research at a whole-brain scale with cellular resolution. Researchers can monitor and manipulate whole-brain neuronal activity in awake and even behaving larvae, providing a new research paradigm for dissecting neural mechanisms of animal behavior. In the field, the next critical step is to uncover the underlying neural circuits. Over the past two decades, virus-based tracing tools have revolutionized the structural analysis of neural circuits. However, the application of these tools in zebrafish research has remained limited due to high toxicity and very low tracing efficiency (~ 1 input per starter cell for the best protocol), which prevents their broader use in zebrafish studies. Therefore, the field is still waiting for effective and applicable tailored methodologies for tracing neural circuits in larval zebrafish.
This study optimized various factors, including the RV strain, RV glycoprotein (G protein) type, G protein expression strategy, and working temperature. It determined that a combination based on the CVS strain yielded optimal results. This optimized combination significantly improved neural circuit tracing efficiency, with an approximately 20-fold higher efficiency than previous reports. It was also easily applicable in zebrafish. The researchers also found that the optimized combination exhibited lower cytotoxicity in infected cells, allowing observations to be extended up to 3-week-old larvae while maintaining the functional activity of infected neurons. This covers the critical time window for whole-brain functional imaging studies in zebrafish. Additionally, this work performed the first functional validation of RV spread in zebrafish, demonstrating its retrograde and monosynaptic specificity in tracing central nervous circuits in larval zebrafish. Furthermore, the researchers developed a transgenic reporter framework using Cre-expressing RV virus, enabling genetic control and vibrant visualization of traced inputs. This allows for cell type-specific tracing of inputs and facilitates the reconstruction of traced neural circuits based on single-neuron morphology.
As an application demonstration, the researchers applied this method to map the monosynaptic inputs to Purkinje cells (PCs) from granule cells (GCs) in the cerebellum of larval zebrafish. They reconstructed the cellular-resolution inputs of PCs and revealed two novel features of the cerebellar circuit: an ipsilateral preference of GC-to-PC connections and subtype specificity of GC-to-PC connections.
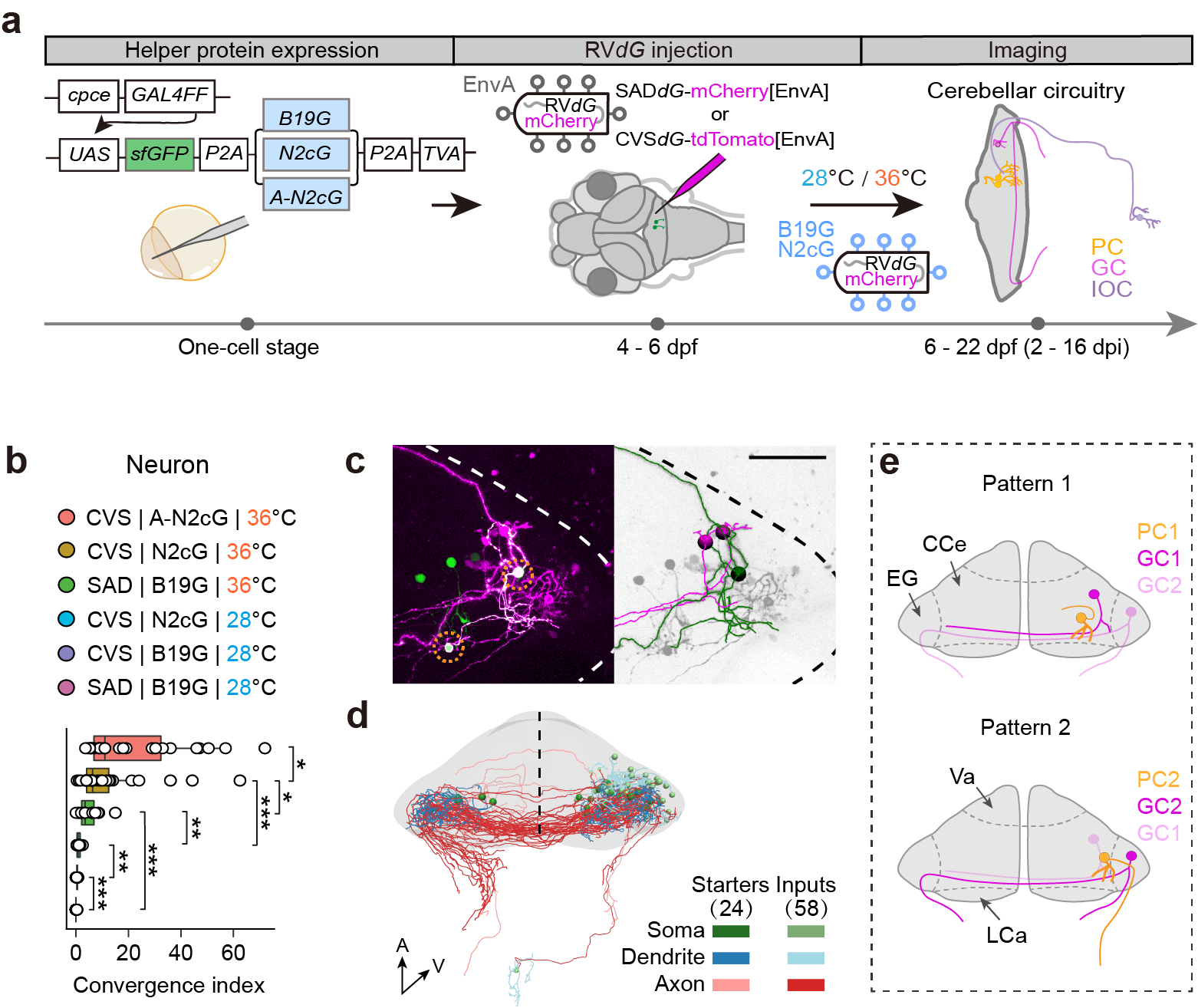
Figure: a, Schematic of the method used to trace inputs to PCs with recombinant RV in larval zebrafish. b, Convergence index (CI) for transneuronally traced neurons to starter PCs. c, Connectivity map between cerebellar PCs and GCs. d, Schematic of the subtype-specific connectivity patterns from GCs to PCs.
This study achieved genetically controllable, efficient, low-toxicity, and highly feasible retrograde trans-monosynaptic tracing in larval zebrafish. Its effectiveness and applicability overcome long-standing technical challenges in the field and will facilitate the structural and functional dissection of neural circuits in larval zebrafish.
This work, entitled " An applicable and efficient retrograde monosynaptic circuit mapping tool for larval zebrafish", was published online in eLife on August 21, 2024.
This study was mainly conducted by CHEN Tianlun and DENG Qiusui, under the supervised of Dr. DU Xufei and Dr. DU Jiulin. Dr. LIN Kunzhang from the Shenzhen Institute of Advanced Technology of the Chinese Academy of Sciences provided the recombinant RV virus; ZHENG Xiudan, Wang Xin, ZHONG Yongwei, and NING Xinyu from the Facility of Mapping Brain-wide Mesoscale Connectome made important contributions. Dr. LI Ying from the Chinese Institute for Brain Research and Dr. XU Fuqiang from the Shenzhen Institute of Advanced Technology of the Chinese Academy of Sciences provided valuable assistance. This work was supported by the Ministry of Science and Technology, the Chinese Academy of Sciences, the National Natural Science Foundation of China, and the Shanghai Municipal Science and Technology Major Project.
Contact:
DU Xufei
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
xufeidu@ion.ac.cn
Keywords:
Viral circuit tracing tool; Recombinant rabies virus; Larval zebrafish
 附件下载:
附件下载: