Time:2025-07-10
The PFC in primates including human has dramatically expanded over the course of evolution, which is believed to be the structural basis underlying high cognitive functions. Previous studies of PFC connectivity in non-human primates have mainly relied on population-level viral tracing and functional magnetic resonance imaging (fMRI). These methods in general lacked single-cell resolution to examine projection diversity. Meanwhile, whole-brain imaging data for tracing axons in the primate brain are massive in size, with macaque brain datasets approaching petabyte (PB) in scale (~1000 terabytes [TB]). How to accurately and efficiently reconstruct single-neuron projectomes from large-scale optical imaging datasets poses a major challenge for the study of mesoscopic connectomes in the primate brains.
On July 10, 2025, Cell published a research article entitled “Single-neuron projectomes of macaque prefrontal cortex reveal refined axon targeting and arborization” by the team from the Center for Excellence in Brain Science and Intelligence Technology (CEBSIT) at the Chinese Academy of Sciences (CAS), in collaboration with the team from the HUST-Suzhou Institute for Brainsmatics. This study reports the first comprehensive study of whole-brain projectomes of the macaque prefrontal cortex (PFC) at the single-neuron level, revealing the organization of macaque PFC connectivity. Comparing macaque and mouse PFC single-neuron projectomes, this study revealed highly refined axon targeting and arborization in primates. These findings not only enhanced our understanding of the neural architecture supporting advanced cognition in primates but also provided crucial insights for inspiring the development of next-generation AI technologies (Figure 1).
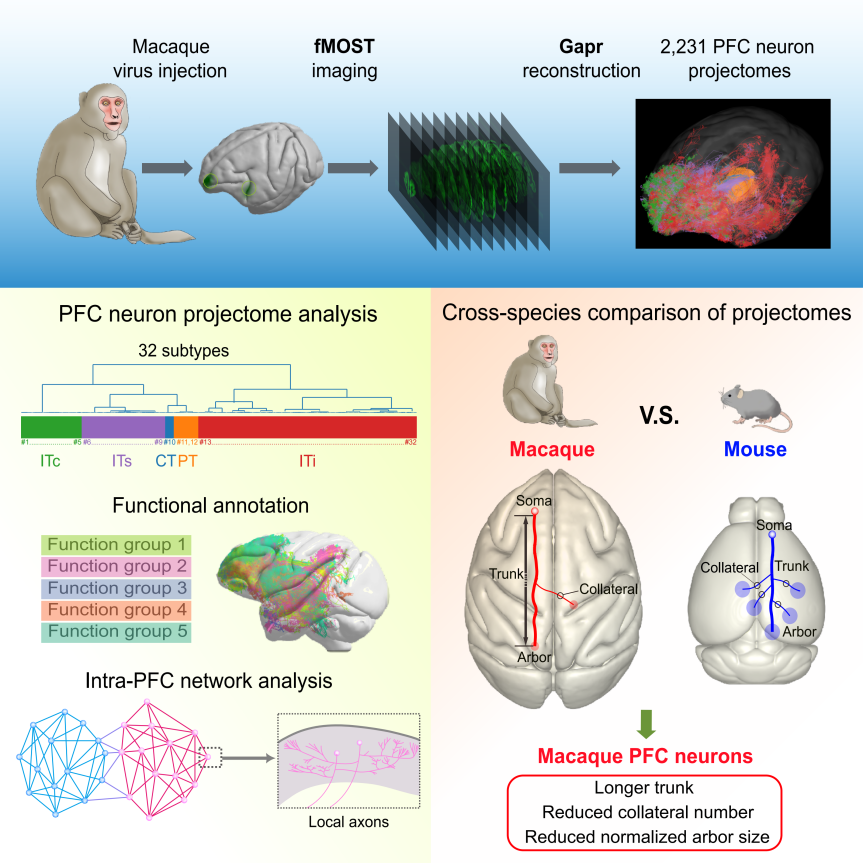
Figure 1. Overview of the study on single-neuron projectomes of the macaque PFC.
To overcome this problem, the research team led by Dr. Jun Yan at the CAS-CEBSIT previously developed Fast Neurite Tracer (FNT), a software tool for single-neuron reconstruction, which enabled the reconstruction of single-neurons projectomes in mouse PFC from TB-sized optical imaging datasets (Gao et al., 2022, 2023). The same team later developed a high throughput single-neuron reconstruction system called Gapr (Gapr accelerates projectome reconstruction) to integrate PB-sized data processing, AI-algorithm-based automatic reconstruction and large-scale collaborative proofreading, laying the groundwork for mapping single-neuron projectomes in the primate brains (Gou et al., 2024).
In this study, researchers performed sparse labeling of single neurons in 19 subregions of the prefrontal cortex in macaques (Macaca fascicularis) by viral infection, and conducted fluorescence micro-optical sectioning tomography (fMOST) imaging. This was followed by large-scale three-dimensional (3D) reconstruction of whole-brain axon projectomes using Gapr system, yielding 2,231 single-neuron projectomes of the macaque PFC (Figure 2). Based on axonal morphology in the whole brain, these neurons were classified into 32 projection subtypes, each exhibiting distinct projection pattern targeting different brain regions including the parietal and temporal cortices, contralateral hemisphere, striatum, thalamus, midbrain, and pons. Further AI-based functional predictions suggested that these projection subtypes were closely associated with sensory, motor, emotional, cognitive, and memory-related higher-order functions (Figure 3).
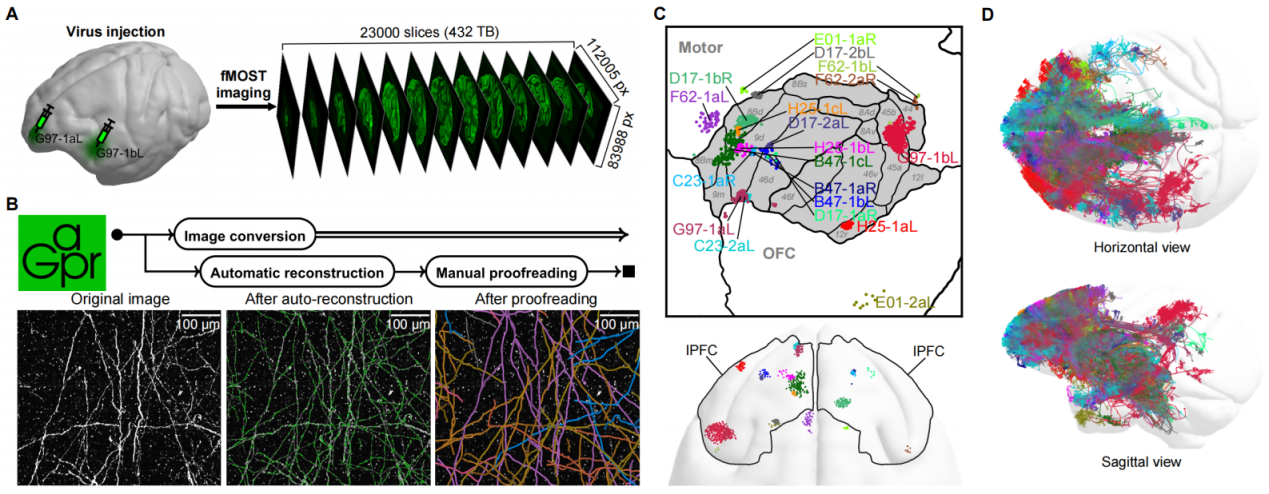
Figure 2. Workflow of data acquisition and visualization for the macaque PFC single-neuron projectome. (A) Acquisition of whole-brain imaging data of the macaque PFC. (B) Utilization of Gapr to perform PB-scale image conversion, automatic single-neuron reconstruction and large-scale collaborative online proofreading. (C) Distribution of injection sites. (D) Distribution of whole-brain projections.
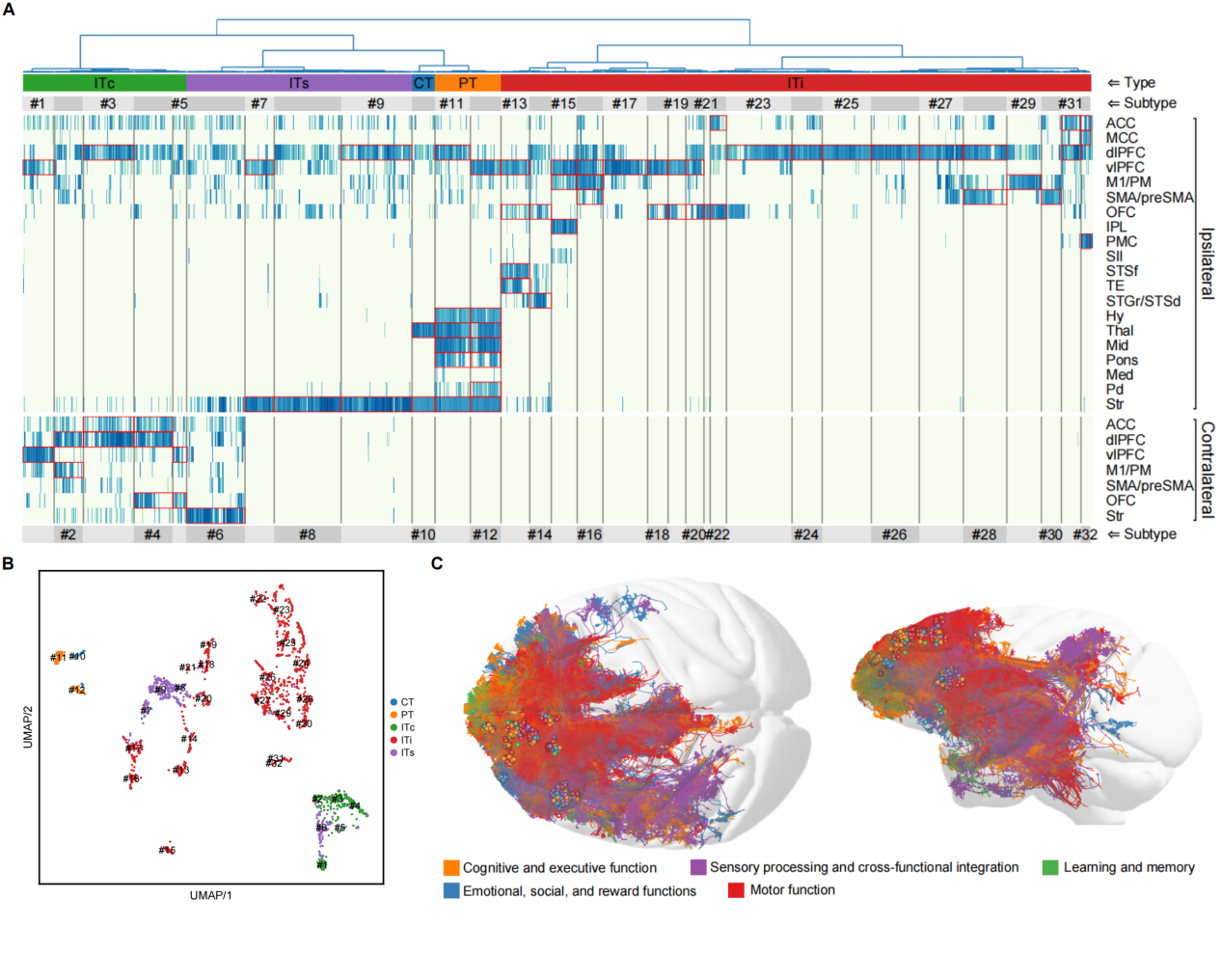
Figure 3. Clustering analysis of projection subtypes and prediction of brain functions for the single-neuron projectome of the macaque PFC. (A) Clustering of 32 projection subtypes. (B) UMAP plot of the 32 projection subtypes. (C) AI-based functional prediction of projectomes.
In-depth analysis of single-neuron projectomes in the macaque PFC uncovered a set of organizational principles (Figure 4). First, distinct PFC neuron subtypes projected to either the parietal or temporal lobes, and neurons in different PFC subregions projected to different subregions within these targets. Second, the macaque PFC exhibited a modular network of intra-PFC connections, which may provide a structural basis for working memory. Third, patchy terminal arbors were found in the PFC projections to the striatum and contralateral cortex in macaque but not in mouse, highlighting more spatially refined innervation pattern in the primate brain. Fourth, macaque PFC neurons with bilateral projections showed a stronger preference of contralateral targeting compared to those in mice, suggesting functional specialization of neurons projecting to the contralateral hemisphere in primates. Finally, the PFC neurons projecting to subcortical areas displayed a topographic relationship between their somata with their targets, suggesting differential downstream innervations across different prefrontal regions.
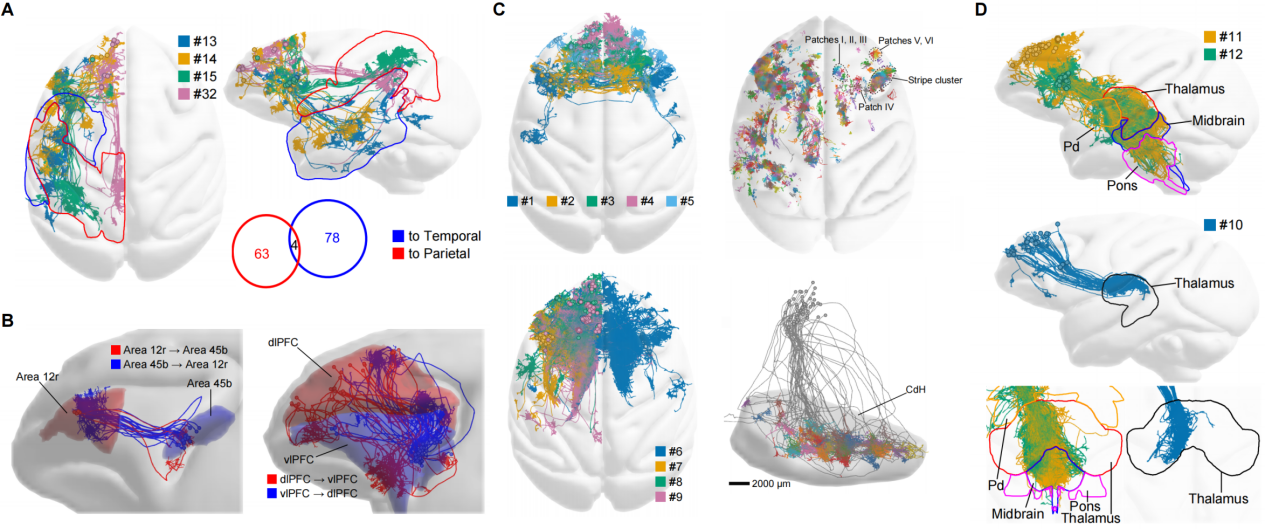
Figure 4. Distinct connectivity patterns in the single-neuron projectomes of the macaque PFC. (A) Projections to parietal and temporal cortices. (B) Intrinsic connectivity among PFC modules. (C) Contralaterally projecting neurons (top left), representative contralateral axon terminal arbors (top right), striatum-projecting neurons (bottom left), and patchy axon terminals formed within the striatum (bottom right). (D) Projections to subcortical areas including the thalamus, midbrain, pons, etc.
A systematic comparison of the single-neuron projectomes between macaque and mouse PFC showed that macaque PFC neurons shared similar target specificity but exhibited distinct morphological features including longer axon trunks, fewer collateral branches, and relatively smaller axon terminal arbors (Figure 5). These results indicated that, compared to rodent neurons, primate neurons possessed simpler structures, more restricted projection targets, and more spatially refined innervation pattern. Such highly modular and selected connectivity of the primate PFC may provide the structural foundation for the emergence of advanced cognitive and executive functions in primate brains.
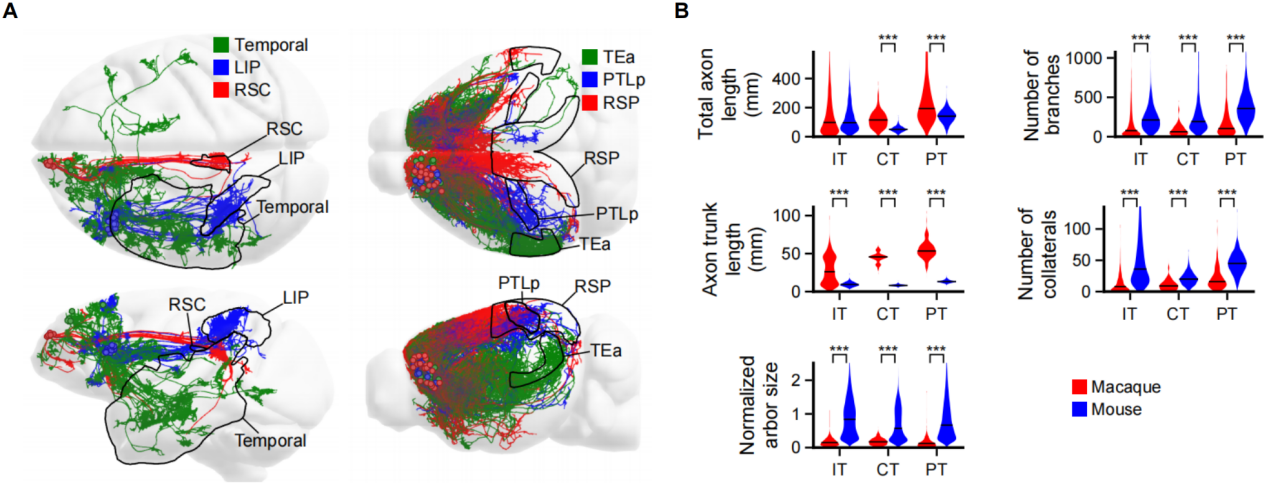
Figure 5. Cross-species comparison of PFC single-neuron projectomes between macaques and mice. (A) Comparison of long-range cortical projection neurons between macaques and mice. (B) Comparative analysis of axon length, number of branches, main axon trunk length, number of collateral branches, and brain-size-normalized axon terminal arbor size across different projection types of neurons.
In sum, this study revealed the structural basis underlying the evolution of higher cognitive functions in the primate brains and provided important clues for exploring the neural origins of psychiatric disorders in human brain, and may inspire new design of artificial intelligence.
This study was led by the team in Center for Excellence in Brain Science and Intelligence Technology (CEBSIT) at the Chinese Academy of Sciences (CAS), in collaboration with the team from the HUST-Suzhou Institute for Brainsmatics and other institutions. Dr. Lingfeng Gou and PhD candidate Yanzhi Wang from Dr. Jun Yan's lab at the CAS-CEBSIT are the co-first authors of this paper, and Drs. Jun Yan, Chun Xu, and Zhiming Shen, as well as Dr. Xiaoquan Yang from the HUST-Suzhou Institute for Brainsmatics, are the co-corresponding authors. This study was from the Mesoscopic Brain Mapping Consortium and was supported by the National Science and Technology Innovation 2030 Major Program, the Lingang Laboratory grant, the National Science Foundation for Young Scientists of China grant, the National Key R&D Program of China, and the CAS Project for Young Scientists in Basic Research.
Contact:
YAN Jun
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
junyan@ion.ac.cn
 附件下载:
附件下载: